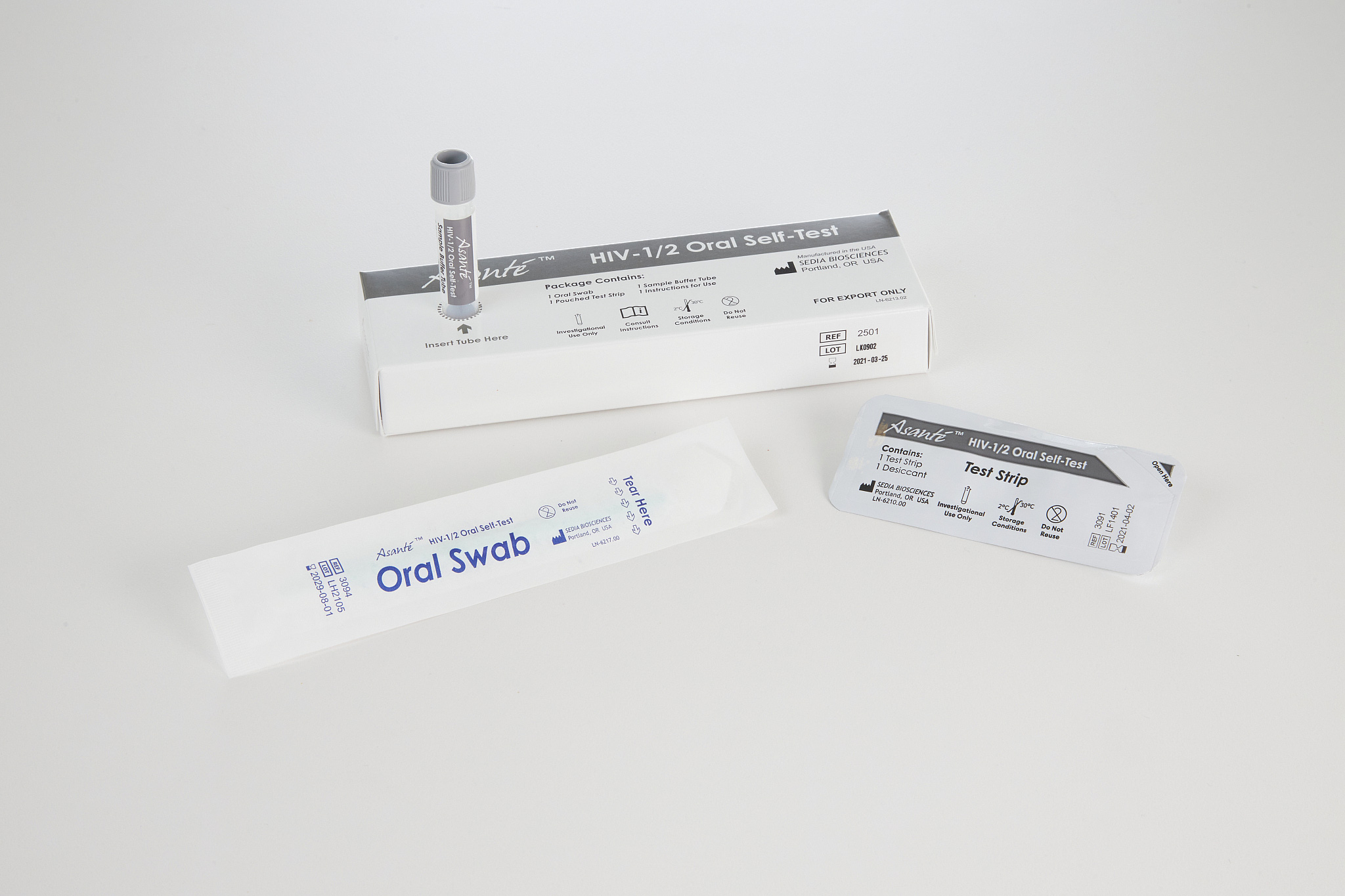
Asanté ® HIV-1/2 ORAL SELF-TEST
IN DEVELOPMENT
FOR THE QUALITATIVE DETECTION OF ANTIBODIES TO TYPE 1 AND TYPE 2 HIV IN ORAL FLUID SPECIMENS
Asanté ® HIV-1/2 Oral Self-Test
A rapid, visually read, in vitro immunoassay for the qualitative detection of antibodies to HIV-1 and HIV-2 in oral fluid specimens. Will be intended for use outside the United States.
Easy to Use
No needles or sharps containers required.
Easy to follow color reference guides in product insert.
Swipe the included swab along the gums and mix with Sample Buffer. Discard Swab and add Test Strip to begin the test.
Convenient
No laboratory infrastructure required.
Results in as soon as 20 minutes.
Flexible
Stored and transported at ambient temperatures.
Easily implemented in a variety of healthcare settings.
PRODUCT SPECIFICATIONS
HIV-1/2 Subtype Antibody Detection
Designed to detect antibodies to both HIV-1 and HIV-2.
Two Reaction Lines
Control Line signifies the specimen was properly added and the test functioned properly.
Test Line indicates a presumptive positive or negative result.
EXPECTED PRODUCT APPLICATIONS
May encourage wide-spread testing in all regions of the world.
Will enable at-risk users to continuously monitor HIV status.
FREQUENTLY ASKED QUESTIONS
CONTACT US
Want to know more or have any questions? Send us a message!