Asanté ® DRIED BLOOD COLLECTION STRIPS
FOR THE COLLECTION, PRESERVATION, AND TRANSPORT OF DRIED BLOOD SPECIMENS
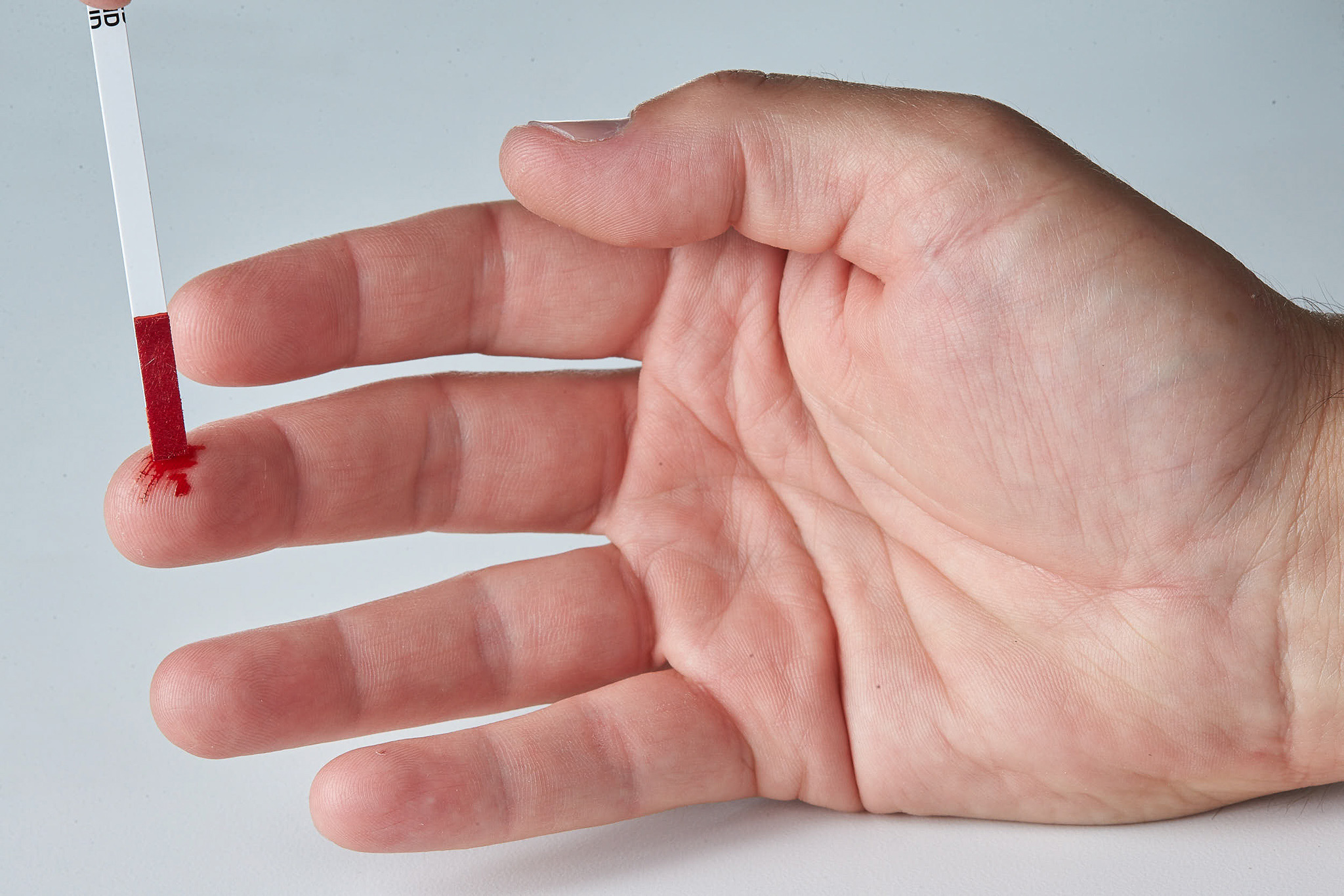
Asanté ® Dried Blood Specimen Collection Strips
Designed to collect and release a defined volume of blood specimen intended as an alternative to the traditional dried blood spot cards.
For Research Use Only: Not for use in diagnostic procedures.
Easy to Use
No puncher or forceps necessary.
Dried specimens in as soon as 20 minutes.
Convenient
Available in 3 sizes to suit different needs.
Can be used with finger stick or previously collected venous blood.
Ideal for high-throughput laboratory processes.
Multi-Use
Use for antibody, enzyme, or protein assays.
Genetic marker screening and long-term biobanking analysis.
Vitamin, hormone, and other biomarkers of research interest.
| Catalog Number | Description |
|---|---|
| 1819-100 | Asanté ® Dried Blood Specimen Collection Strips, 60 mm2 |
| 1820-100 | Asanté ® Dried Blood Specimen Collection Strips, 100 mm2 |
| 1821-100 | Asanté ® Dried Blood Specimen Collection Strips, 200 mm2 |
MORE PRODUCT FEATURES AND DATA
Ideal Alternative to Classical Dried Blood Spot Cards for High Throughput Sampling and Testing
Asanté ® Dried Blood Specimen Collection Strips facilitate high throughput sampling and testing, overcoming many of the shortcomings of classical dried blood spot cards. The diagram below shows how the Asanté ® Strips can be used when collecting specimens that must later be tested in a microtiter plate (such as the 8 x 12 or 96-well array typically used for ELISA’s).
| Catalog Number | Dimensions | Specimen Absorbed |
|---|---|---|
| 1819-100 | 5 x 12 mm | ~16 μL |
| 1820-100 | 5 x 20 mm | ~27 μL |
| 1821-100 | 10 x 20 mm | ~54 μL |
FREQUENTLY ASKED QUESTIONS
CONTACT US
Want to know more or have any questions? Send us a message!