Asanté ® HIV-1 RAPID RECENCY ® ASSAY
FOR DISTINGUISHING RECENT VS LONG TERM HIV-1 INFECTIONS IN POSITIVE HIV-1 SPECIMENS
Asanté ® HIV-1 Rapid Recency ® Assay
A rapid, 20 minute in vitro immunoassay that differentiates recent from long-term HIV-1 infections for real time surveillance of new HIV-1 infections and the estimation of HIV-1 incidence rates in a population.* The assay can be used with blood (both venous and fingerstick), serum, or plasma specimens as either a laboratory or point-of-collection test.
For Research Use Only: Not for use in diagnostic procedures.
Easy to Use
The Asanté ® HIV-1 Rapid Recency ® Assay is faster, more cost effective and simpler to use than conventional HIV-1 incidence lab assays.
Add a loopful of specimen and the Test Strip to the Sample Buffer to begin the assay.
Low False Recency Rate
False recency rate comparable to the Sedia ® HIV-1 Limiting Antigen (LAg)-Avidity EIA laboratory assay.*
Convenient
No laboratory infrastructure necessary. This enables field testing and collection of recency data in areas that are resource constrained.
| Catalog Number | Description |
|---|---|
| 1130-020 | Asanté ® HIV-1 Rapid Recency ® Assay, 20 Tests, For Research Use Only |
| 1130-100 | Asanté ® HIV-1 Rapid Recency ® Assay, 100 Tests, For Research Use Only |
| READER | For additional details regarding the Asanté ® Test Strip Reader contact us today! |
PRODUCT SPECIFICATIONS
| Summary of Specifications | |
|---|---|
| Assay Time | 20 minutes |
| Processing Temperature | 15° to 37°C (59° to 98.6°F) |
| Storage Temperature | 2° to 30°C (35.6° to 86°F) |
| Specimen Type | Human whole blood, plasma (in EDTA or ACD anticoagulants), or serum |
| Specimen Volume | One loopful or 5 µL if using pipette |
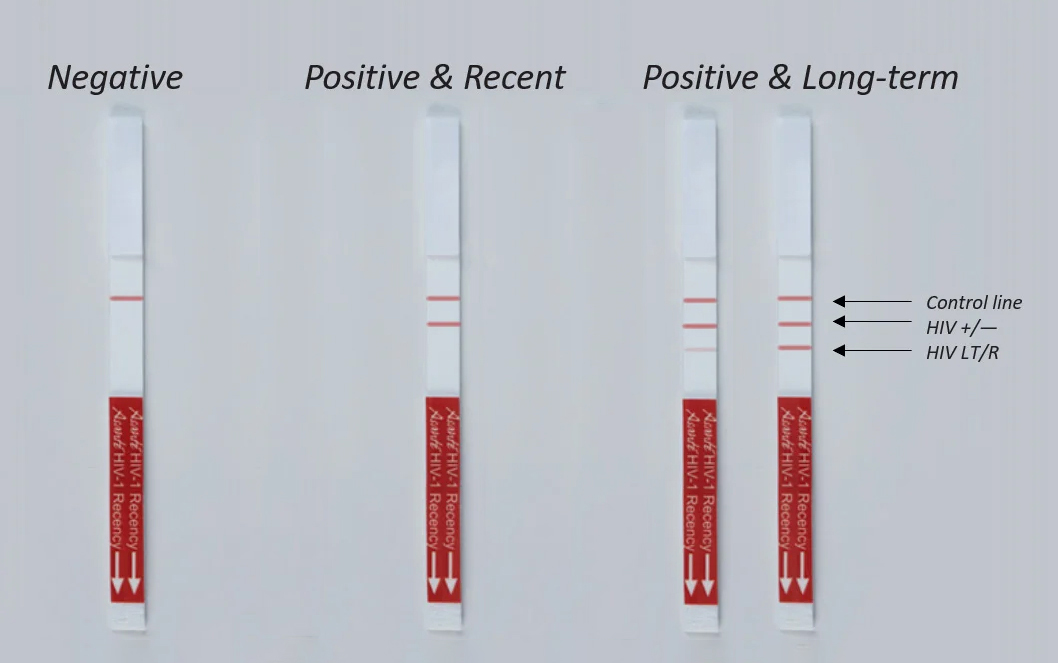
| Result Line 1 | Control; ensures proper functioning of the test |
| Result Line 2 | Verification; ensures exclusion of HIV negative specimens |
| Result Line 3 | LT/R; differentiates recent from long term infection |
| Custom Antigen | Custom, multi-clade CDC “rIDR-M” HIV-1 gp41 antigen that provides for reduced HIV-1 subtype bias |
| Mean Duration of Recent Infection | Approximately 180 days* |
| U.S. CDC Qualified | Each lot is performance tested by the U.S. Centers for Disease Control prior to market release |
| Tests per Kit | Available in configurations of 20 tests or 100 tests per kit |
| For Research Use Only | Not for use in diagnostic procedures, RUO products are not to be used for diagnostic purposes, patient management, clinical purposes, or for investigational use within the U.S. |
U.S. CDC Tested
Each Asanté ® HIV-1 Rapid Recency ® Assay lot is performance tested by both Sedia’s Quality Control Department and by the U.S. Centers for Disease Control prior to market release.
Certification of CDC testing can be provided upon request.
Contains Three Reaction Lines
Control line indicates the specimen was properly added and the test functioned properly.
Verification line ensures HIV-negative specimens are excluded.
LT/R line differentiates recent from long-term specimens.
Custom Antigen
The assay incorporates a custom multi-clade CDC “rIDR-M” HIV-1 gp41 antigen that provides for reduced HIV-1 subtype bias. Mean duration of recent infection approximately 180 days.*
PRODUCT APPLICATIONS
Enable real time surveillance of new HIV-1 infections and inform resource management.
Evaluate the effectiveness of HIV intervention programs.
Identify optimal populations at risk for targeting HIV vaccine clinical trials. Monitor effectiveness of vaccine candidates.
Identify new “hot spots” of infection.
FREQUENTLY ASKED QUESTIONS
CONTACT US
Want to know more or have any questions? Send us a message!